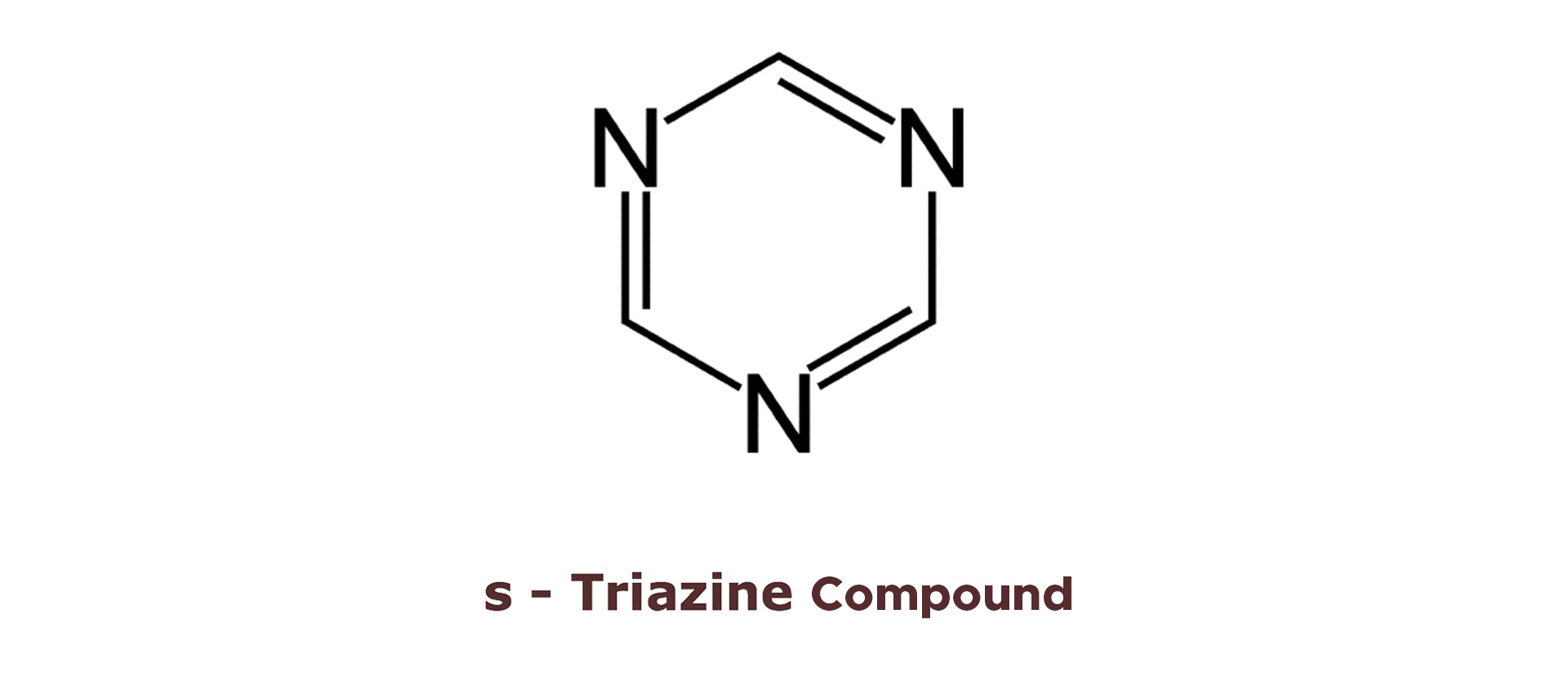
Triazines have a variety of pharmacological properties including anticancer, antimicrobial, antimycobacterial, anti-HIV, etc. Besides this, they are also used as renowned agricultural chemicals such as simazine, aitrazine, propazine, etc. Trazines can be symmetrical or asymmetrical depending on the position of nitrogen atom in the ring. If nitrogen is on the 1, 3 and 5 position, then it is known as s-triazine. Different methods have been reported on the preparation of triazines from different starting materials. Among them, the preparation of triazine derivative using 2,4,6-trichloro-1,3,5-triazine, which is also known as cyanuric chloride as starting material, seemed to be the best option to achieve our desired products.
Cyanuric chloride is a commercially available cheap reagent. It is a dusty white solid with a very strong smell and is soluble in most organic solvents. Triazine has a very unique property of being able to replace its three chlorine atoms at different temperatures. The first chlorine atom can be replaced at 0-50C, second can be replaced at room temperature and third can be replaced at higher temperatures above 1000C.
Cyanuric chloride is a commercially available cheap reagent. It is a dusty white solid with a very strong smell and is soluble in most organic solvents. Triazine has a very unique property of being able to replace its three chlorine atoms at different temperatures. The first chlorine atom can be replaced at 0-50C, second can be replaced at room temperature and third can be replaced at higher temperatures above 1000C.
Commonly nucleophilic substitution does not occur in aromatic systems due to the high electronic density of the aromatic ring. However, in the case of cyanuric chloride, the nitrogen atom on the ring strongly attracts the electronic clouds, and chlorine atoms in the position 1, 3 and 5 exert a negative inductive effect draining electronic charge from the ring. Because of the above, positions 2, 4 and 6 of cyanuric chloride become suitable for nucleophilic substitution. As chlorines are exchanged for other side chains the aromatic ring (depending on the substituent introduced) increases its electronic density due to the loss of the negative inductive contribution of chlorine, making it more difficult to exchange the next chlorine atom. For that reason, the activation energy of the first substitution is lower than the second which is in turn lower than the third.
Dr. Jyotindra Mahyavabshi
Department of Chemistry
Shrimad Rajchandra Vidyapeeth.